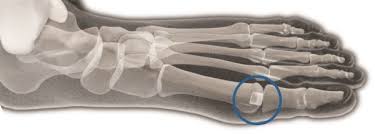
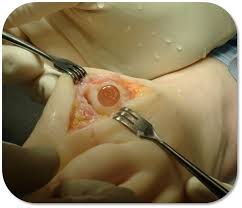
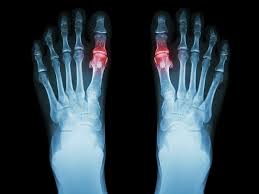
WHAT IS THE CARTIVA® SCI?
The Cartiva® Synthetic Cartilage Implant (Cartiva SCI) is a man-made (synthetic) implant that is made of a soft plastic-like substance (polyvinyl alcohol) and salt water (saline). These materials are combined and molded into a solid, slippery and durable implant.
WHAT IS THE CARTIVA® SCI USED TO TREAT?
The Cartiva SCI is intended to treat painful arthritis in the joint of the big toe ( rst metatarsophalangeal joint). This arthritis of the big toe, also known as osteoarthritis or “OA”, involves the wearing down of the cartilage tissue located in the big toe joint. The worn down cartilage can cause pain.
WHAT YOU NEED TO KNOW
WHO SHOULD NOT RECEIVE THE CARTIVA® SCI (CONTRAINDICATIONS)?
- Tell your doctor if you think you have an infection in your foot. An infection makes it risky to have the Cartiva SCI. You might need another surgery to remove it because infections near the implant are hard to treat. Your doctor should not implant this device in you if you have an infection. (It is not allowed for use in patients with infections).
- Tell your doctor if you think you have ever had any allergy to or reacted to any plastic or an implant. The Cartiva SCI is made from a plastic-like mixture (polyvinyl alcohol and saline). You could be allergic to it. An allergic reaction to the Cartiva SCI might mean you would need more surgery to remove it. Your doctor should not implant this device in you if you might be allergic to it. (It is not allowed for use in patients who are allergic to polyvinyl alcohol or saline.)
- Tell your doctor if you have a form of arthritis called gout that also causes small lumps (tophi) to form under the skin around your joints. The Cartiva SCI might not work in your joint with this kind of arthritis. Your doctor should not implant this device in you if you have gout with tophi.
- Tell your doctor if you have any of the following conditions that can hurt implant support. - You had cancer
- You had a hip dislocation
- You have brittle bone or bone that breaks easily
- You have taken a steroid medication in the past
- You had an organ transplant
- You have taken a medication called an immunosuppressant in the past - You have a history of any growths (tumors) in your bones
These conditions might lead to changes in your bone that might make the Cartiva SCI device unable to work properly.
You should speak to your doctor to determine if the above conditions apply to you, or if other conditions may make the Cartiva SCI not right for you.
- HOW HAVE WE TESTED THE CARTIVA SCI® IN CLINICAL TRIALS?
- A controlled clinical study tested the Cartiva SCI. The study happened in hospitals in Canada and the United Kingdom. Patients had OA of the rst MTP joint, similar to you. Study patients received the Cartiva SCI or a fusion of their rst MTP joint. 202 patients were treated in this study. 152 patients received the Cartiva SCI implant. 50 patients had fusion surgery. Patients were seen over a two-year period from surgery including a visit two years after surgery. Of the Cartiva patients, 151 patients of the 152 were available for the two year visit and 47 of the 50 fusion patients were available at two years. The study results
were reported to the U.S. Food and Drug Administration (FDA). - The patients with the Cartiva SCI implant saw similar outcomes to the patients with the fusion treatment. In the clinical study, 89 out of every 100 Cartiva SCI patients had signi cant pain relief at two years after treatment. 98 out of every 100 Cartiva SCI patients maintained or improved their function at two years after treatment.
- 74 of every 100 Cartiva SCI patients maintained or improved their amount of motion at two years after treatment. People with a fusion surgery were unable to keep their motion. This motion in the Cartiva SCI patients did not cause better function in day-to-day activities.
The Cartiva SCI and fusion groups had similar improvements in function at two years.
HOW LONG CAN I EXPECT THE CARTIVA SCI® TO LAST?
The Cartiva SCI device is a long-term treatment for your big toe joint. There have been limited cases where the Cartiva SCI was removed because a patient still had pain in their big toe joint. In the study, 9 out of every 100 Cartiva SCI subjects had the device removed within 2 years after surgery. In these cases, the patient’s joint was fused with plates or screws (arthrodesis). The use of the Cartiva SCI device did not limit the patient’s options for a successful fusion.
- A controlled clinical study tested the Cartiva SCI. The study happened in hospitals in Canada and the United Kingdom. Patients had OA of the rst MTP joint, similar to you. Study patients received the Cartiva SCI or a fusion of their rst MTP joint. 202 patients were treated in this study. 152 patients received the Cartiva SCI implant. 50 patients had fusion surgery. Patients were seen over a two-year period from surgery including a visit two years after surgery. Of the Cartiva patients, 151 patients of the 152 were available for the two year visit and 47 of the 50 fusion patients were available at two years. The study results
ARE THERE ALTERNATIVES TO USING THE CARTIVA® SCI?
Surgery will likely be recommended by your doctor if other non-operative methods have not been successful at reducing your big toe arthritis pain. You may wish to ask your doctor about any other possible treatments for your big toe arthritis pain.
Other surgical treatment options may include:
• Cheilectomy: A surgery that involves shaving bone from both the joint surfaces of your big toe
and removal of the diseased portion of the metatarsal head.
• Hemi-arthroplasty: A surgery that replaces part of your joint with metal or plastic parts to serve as the new surface of the rst metatarsophalangeal head.
• Total Joint Replacement: A surgery that replaces your joint with metal and plastic parts to replace both sides of the MTP joint.
• Fusion (arthrodesis): A surgery where the two sides of the MTP joint are cleared of cartilage. The two bones are held together with plates and/or screws so that the bones grow together. Fusion was studied and compared to the results for patients implanted with the Cartiva device, as discussed above.
Your doctor will have more information on each of these options and other possible treatments, as well as the bene ts and risks for each of the treatment options.
WHAT PROBLEMS HAPPENED FROM CARTIVA® SURGERY?
The Cartiva SCI device study followed 152 patients for 2 years after surgery.
The most common adverse events were:
Pain due to surgical procedure
Wound swelling, draining or delayed healing or scarring
Joint stiffness or hardening of your joint (induration)
Tendon swelling (in ammation)
Damage to nearby nerves, arteries or veins
Infection
Numbness in toes
Changes in the way you walk (gait disturbance)
Blood clot formation in one or more of the deep veins in your body (deep vein thrombosis), collection of uid in the lungs (pulmonary embolism), or blood clot (thrombosis) formation in other vessels
Reactions to the drugs or anesthesia (the medicine they used to put you to sleep) used during and after surgery
Heart attack
Blood loss, blood vessel damage, swelling of the blood vessel in your leg (phlebitis) or a localized collection of blood outside the blood vessels (hematoma)
Additional surgery to remove or replace the implant due to more pain
Other operative procedures
Pain and discomfort associated with the operative site or presence of implants
Joint with excess motion (instability) or at an abnormal angle (malalignment)
Swelling or escape of uid in body cavity (effusion)
Fracture of part of your sesamoid or metatarsal bone
Progressive osteoarthritis or disease of the joint (arthropathy)
Changes to the foot bone
Implant may loosen, wear out, or break which may need another operation to remove the implant and may need another method of treatment
Sensitivity or allergy to the implant material Bone loss
Poor positioning of the implant
Joint or bone irritation
Damage to surrounding tissues Other unexpected reactions